The Benefits of Low Endotoxin Products
by Kathy Miscioscio
What are Endotoxins and why are they important?
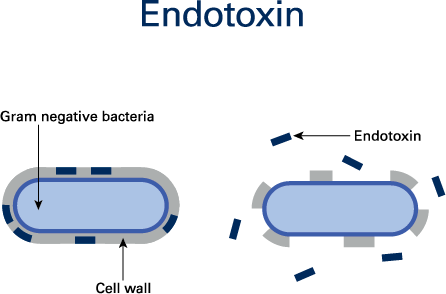
Bacterial endotoxins are released from the outer shell membranes of decaying Gram-negative bacteria. As a type of pyrogen, bacterial endotoxins produce fever in humans and animals and can pose l
ife-threatening risks to patients (Figure 1).
“Microbial pyrogen” as opposed to “Gram-negative bacterial endotoxin” has become a general descriptive term for many different substances. However, pyrogenic substances can be produced by some Gram-positive bacteria, mycobacteria, fungi and also viruses, but the pyrogens produced by Gram-negative bacteria are of significance to the pharmaceutical industry.
Bacterial endotoxins are members of a class of phospholipids called lipopolysaccharides (LPS). The release of LPS from bacteria takes place after death and bursting of the cell wall. Examples of endotoxin-releasing Gram-negative bacteria are Escherichia coli, Proteus, Pseudomonas, Enterobacter and Klebsiella.1
Manufacturers of pharmaceuticals and medical devices are required to test their products for endotoxin levels. The presence of pyrogens is a critical safety concern since these substances cannot be easily removed. Unlike viable microbial contaminants which can be destroyed by various sterilization techniques, pyrogens are difficult to remove and deactivate.
The Importance of Low Endotoxin Consumable Products
The FDA sets the endotoxin limits for pharmaceutical products produced in the US or imported into the US. The limits for endotoxins are stated in USP chapter <161>, Transfusion and infusion assemblies and similar medical devices. The USP requirement for medical devices specifies a limit of 0.5 EU/mL
(endotoxin unit/mL) or 20 EU/device for products that directly or indirectly contact the cardiovascular and lymphatic systems. The limit for products in contact with cerebrospinal fluid is 0.06 EU/ mL or 2.15 EU/device.2
The US FDA states that “It is difficult to remove endotoxins from products once present. It is far better to keep finished products and components relatively endotoxin-free rather than have to remove it once present.”1 Therefore, it is important to control the level of endotoxin units in materials used in the production of drug products and medical devices.
Endotoxins can be introduced into a product or process by operators, raw materials, secondary materials, and solvents, such as cleaning products, that are used in the formulation or manufacture of pharmaceuticals. A source material of particular concern for endotoxin levels is water. Water for Injection (WFI) Water for Injection (WFI) is used in the production of parenteral drugs and other critical products when endotoxin levels must be controlled. The limits for endotoxins in WFI are stated in the USP Monograph on Water for Injection3 and the European Pharmacopoeia (Ph. Europe), Water for Injection Monograph4.
Purified water can be used in the production of non-parenteral preparations and other activities such as cleaning of equipment. Source water can be purified through several means, such as deionization (DI) and reverse osmosis (RO). The chart (Figure 2) compares the specification of Purified Water with US and European WFI specifications.
WFI or Low Endotoxin Certified?
There are no regulations for endotoxin levels in cleanroom wipes and alcohol products. However, convention is that low endotoxin products meet the USP requirement of <0.5 EU/ml or <20 EU/device. Products certified as low endotoxin are not required to use low endotoxin ingredients, such as WFI, but the final product must be tested for conformance to USP requirements for endotoxin levels. Finished products containing WFI have a component (water) that is
low endotoxin, but for the finished product to be classified as low endotoxin, it must be tested to meet the stated limits to ensure all components of the product, packaging, and manufacturing cycles have been controlled to produce a low endotoxin result.
Summary
As stated earlier, it is much more effective to prevent the introduction of endotoxins into processes and products than it is to remove them downstream. The use of low endotoxin products can minimize the risk of endotoxin contamination of a pharmaceutical product, thus minimizing the risk of adverse patient reaction to intravenous (injected) medicines. To help achieve that goal, Contec makes a full line of low endotoxin certified products, including presaturated wipes, dry knit and nonwoven wipes, hydrogen peroxide and sterile 70% isopropyl alcohol, for the most critical applications. Each lot is tested before release to ensure a low level of endotoxin.
References
1 FDA Inspection Technical Guides > Bacterial Endotoxins/Pyrogens, Date: 3/20/85 Number: 40
2 USP chapter <161>, Transfusion and infusion assemblies and similar medical devices
3 USP Monograph, Water for Injection
4 European Pharmacopoeia, Water for Injection Monograph (0169)
